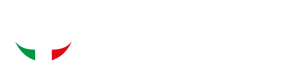Implant-prosthetic rehabilitation of the atrophic posterior mandible with additively manufactured custom-made subperiosteal implants: a cohort study
L. A. Vaira, A. Biglio, A. Favro, G. Salzano, V. Abbate, J. R. Lechien, G. De Riu: Implant-prosthetic rehabilitation of the atrophic posterior mandible with additively manufactured custom-made subperiosteal implants: a cohort study.
Abstract. The aim of this study was to retrospectively analyse a series of patients with posterior mandibular atrophy rehabilitated with custom-made subperiosteal implants. The study included patients with severe posterior mandibular atrophy who had undergone rehabilitation with subperiosteal implants between September 2018 and August 2022 in the Maxillofacial Surgery Operative Unit of the University Hospital of Sassari. Complications and the success rate were reviewed. Data from 30 implants placed in 17 patients were included and analysed. There were no major complications during the surgeries.
The main postoperative sequela was oedema, which was reported as moderate by most patients and had completely regressed within 10 days of surgery. No partial or complete exposures, infections, or loss of the implants were detected during follow-up (average follow-up 22.5 months). Control computed tomography scans, performed at 6 months and then annually in all cases, did not show significant bone loss below the abutments, displacement of the implants, or loss or loosening of the osteosynthesis screws. Subperiosteal implants may represent a safe and reliable technique for the rehabilitation of severe atrophy of the posterior mandible. Prospective studies with a long follow-up will be needed to establish the long-term results of this type of implant-prosthetic rehabilitation.
The implant-prosthetic rehabilitation of severe atrophy of the posterior mandibular region is a difficult challenge1,2. Guided bone regeneration with autologous and alloplastic grafts currently represents the gold standard for the treatment of this type of condition, as it allows the bone volumes required for correct positioning of the implants to be restored3–5. However, the long rehabilitation times and need for multiple surgeries often make this procedure unacceptable for patients seeking a less biologically burdensome solution.
The failure rate of bone grafts is still significant, ranging from 5% to 20%6 Furthermore, implants inserted in reconstructed bone have a significantly higher failure rate than those positioned in the native bone, which can reach 13% after 1 year7, compared to the 1–3% failure rate for implants placed in native bone8. Only a few graftless techniques are available for the implant-prosthetic rehabilitation of the edentulous posterior mandible.
Short implants are a safe and effective solution, supported by solid scientific evidence, however their use is not applicable if the bone height above the inferior alveolar nerve (IAN) is less than 5 mm or if the bone thickness does not allow the placement of implants of a sufficient diameter9,10. Lateralization of the IAN is another technique by which a sufficient bone height for immediate implant placement can be obtained11,12. However, this is burdened by a significant rate of persistent neurological complications13,14 and is becoming increasingly less popular.
In 2017, Mommaerts15 developed and proposed a new concept of subperiosteal implantology for graftless rehabilitation of the maxilla. This concept made it possible to manufacture custom-made implants using computer- aided design and manufacturing (CAD/ CAM) and laser melting technologies, with a new design that allows rigid fixation of the implant on the maxillary pillars, thereby making it possible to overcome all of the limitations historically associated with this type of rehabilitation. In recent years, an increasing amount of scientific evidence has been published regarding the short- and medium-term safety and efficacy of this new generation of subperiosteal implants for the rehabilitation of the atrophic upper jaw16–22.
The rehabilitation of the posterior mandible with new generation subperiosteal implants is a topic much less addressed in the literature to date, and there are no precise indications regarding the design of the implants, the surgical technique, and the short-, medium-, and long-term outcomes. The aim of this study was to retrospectively analyse a series of patients with posterior mandibular atrophy who were rehabilitated with additively manufactured custom-made subperiosteal implants.
Materials and methods
This study included consecutive patients with severe posterior mandibular atrophy (e.g. Cawood and Howell class V and VI) who underwent rehabilitation with subperiosteal implants between September 2018 and August 2022 in the Maxillofacial Surgery Operative Unit of the University Hospital of Sassari. All patients had been followed up for a minimum of 6 months, during which they had regular clinical and radiological examinations. The study received ethical approval from the Ethics Committee of the University of Sassari (PG/2023/6411). This study has been reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines23
Digital planning
All patients eligible for rehabilitation with subperiosteal implants underwent cone beam computed tomography (CBCT) of the maxilla and mandible (slice thickness 0.1–0.3 mm) with a sufficiently large field of view to include the entire mandible, including the mandibular angles. The case study was completed with the acquisition of digital scans of both dental arches and with the diagnostic wax-up of the prosthetic project necessary to establish the orientation and length of the implant abutments. The prosthetic plan was prepared and executed by the same experienced prosthodontist (A.B.).
The DICOM and STL files were then sent to the company responsible for manufacturing the implant (B&B Dental, San Pietro in Casale, Italy). The DICOM files obtained from the CBCT scan were extracted and imported into B&B Dental GS software (B&B Dental) which allows the bone segments to be reconstructed in three dimensions (3D). The 3D images were cleaned of scattering and other inaccuracies, and the course of the IAN was identified and marked. The STL files of the dental arches and of the diagnostic wax-up were then merged with the 3D model of the jaws.
The 3D files were imported into Meshmixer software (Autodesk, San Rafael, CA, USA) and the implant was designed in accordance with the surgeon’s indications. The positions of the screw holes were determined taking into account the position of the IAN in order to avoid neurological damage during fixation. On the basis of the prosthodontist’s preferences, cementable or multiunit abutments integrated into the structure of the implant were designed and manufactured.
The abutments were always housed in slots created in the alveolar crest so that they could rest on the basal bone, which is less prone to further resorption over time. The length and orientation of the abutments were established in accordance with the diagnostic wax-up and the thickness of the gingiva, as determined by the scan of the arches. The implant was always designed with the goal of keeping the majority of the plate and the fixation screws as far away from the surgical wound as possible.
For this reason, if all of the abutments were behind the emergence of the mental nerve, the implant was made to run below the nerve (Fig. 1). On the lingual side, a connection between the abutments was provided in all cases in which the mylohyoid crest, a limit that cannot be crossed by the implant, was not too superficial. The 3D models of the bones, gums, prostheses, and implants were then re-imported into B&B Dental GS software and submitted for the surgeon’s approval.
Implant manufacturing
After approval of the project by the surgeon, the implant was made in grade V titanium by double laser melting technology (MYSINT100; Sisma, Piovene Rocchette, Italy). The implant then underwent a passage in a sintering oven (Nabertherm GmbH, Lilienthal, Germany) at 840 °C for 4 h and then at 500 °C for a further 2 h to make the metal stable and free of porosity. During this process, the heat distributed over the implant does not induce any dimensional changes. The abutments were then finished with the aid of a 5-axis milling machine (Datron D5; Datron Dynamics Inc., Milford, NH, USA), and the internal threads of the multiunit abutments, if any, were made. The subperiosteal implant was finally washed in the organic acid Dowclene 1601 (Dow Chemical Company, Midland, MI, USA) to eliminate all possible impurities and finally sterilized. The templates for preparing the slots for the housing of the abutments in the alveolar crest were milled in cobalt chrome (Datron D5) in order to be resistant and at the same time thin and easy to position. Finally, a stereolithography model of the jaw was 3D-printed in resin (Stratasys Objet30; Stratasys, Eden Prairie, MN, USA) and provided to the surgeon.
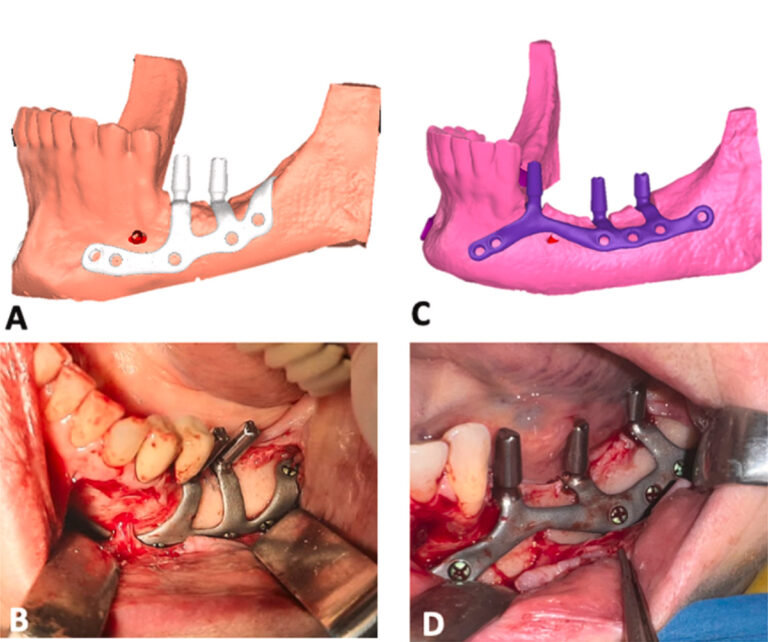
Fig. 1. (A) Digital planning and (B) intraoperative view of a subperiosteal implant that runs below the mental nerve; case 14 (female, age 49 years). (C) Digital planning and (D) intraoperative view of a subperiosteal implant that runs over the mental nerve; case 10 (female, age 65 years).
Surgery
The surgeries were performed by the same surgeon (L.A.V.), under local anaesthesia, without the aid of intravenous sedation. Local anaesthesia was performed with articaine with 1:100,000 epinephrine (Supplementary Material Video S1). An IAN block was not routinely performed as in any posterior jaw implant surgery. The vestibular fornix was infiltrated from the trigone to the incisor region both superficially and deeply, up to the inferior border of the mandible. Local anaesthesia was then completed with infiltration of the mandibular lingual side to cover the entire surgical site.
A full-thickness mucosal incision was made following the course of the edentulous crest, extending mesially, intrasulcularly, or paramarginally on the residual teeth. At the level of the edentulous crest, it is essential that the incision splits the residual keratinized mucosa, often reduced to a strip of a few millimetres, ensuring that at least 2 mm is left on the buccal side so as to provide an adequate lining to the abutments. Two vestibular releases were then performed. The posterior release was performed at least 5 mm from the distal abutment in order to favour the healing of the mucosa around the latter. The position of the anterior release was instead established based on the length of the anterior arm of the implant; this should be more mesial than the nearest screw hole, to reduce the risk of exposure during healing.
After the mucosal incision, a full-thickness buccal flap was elevated, and the mental nerve was identified and preserved. The buccal side of the mandible was extensively skeletonized from the trigone region to the parasymphyseal area, extending inferiorly below the external oblique line towards the lower margin of the mandible. If the course of the implant required it, the mental nerve was isolated at 360° with the aid of a curved dissector. Finally, the periosteum on the lingual side of the mandible was elevated up to the mylohyoid crest, which represents the limit of the dissection.
The cutting guide for ridge preparation was then seated in place and pressed firmly against the bone surface (Fig. 2A). The slots in the alveolar crest for the housing of the abutments were prepared using a cylindrical diamond bur (B&B Dental) with a diameter corresponding to the width of the slot. During the ridge preparation phases, especially if the IAN is very superficial, the patient may feel pain making it necessary to block the IAN. Supplementary material related to this article can be found online at doi:10.1016/j.ijom.2024.01.003.
Once the housings for the implants had been made (Fig. 2B), the subperiosteal implant was inserted. The insetting of implants that run above the mental nerve is simpler and does not require complete isolation of the mental nerve. Otherwise, implant insertion is a bit more complex: the anterior arm is inserted vertically below the nerve and gently pulled forward with forceps. Once the nerve has been passed, the implant can be placed horizontally and moved forward to its final position.
The correct fitting of the implant was then meticulously checked. Any poor fittings are generally related to inaccurate preparation of the housings for the abutments. The housings were finished in these cases before fixing the implant. Once a satisfactory fit was achieved, the implant was pressed firmly into place. Rigid fixation was performed with 2-mm diameter grade V titanium osteosynthesis screws (B&B Dental). In order to avoid damage to the IAN, the length of the screw was decided on the basis of the underlying bone thickness, which was evaluated during the surgical planning; this could vary from 4 mm to 10 mm. The 1.7-mm surgical bur used for the preparation of the holes could be equipped with a stop at a pre-calibrated height. If the screw did not reach sufficient tightening torque, a safety screw with a diameter of 2.3 mm was used. Each implant was fixed with at least four osteosynthesis screws (Fig. 2C). Following completion of the fixation, the mucosa was sutured after releasing the periosteum (Fig. 2D).
All patients were prescribed postoperative antibiotic therapy (amoxicillin + clavulanic acid 1 g twice daily for 6 days) and analgesics. Delivery of the provisional prosthesis took place 10 days after surgery, after the sutures had been removed. The provisional prosthesis was fabricated based on a conventional precision impression and readapted as necessary during the following months to facilitate the conditioning and healing of the soft tissues. The final prosthesis was delivered 6 months after the surgery, once the soft tissues had been conditioned (Fig. 3).
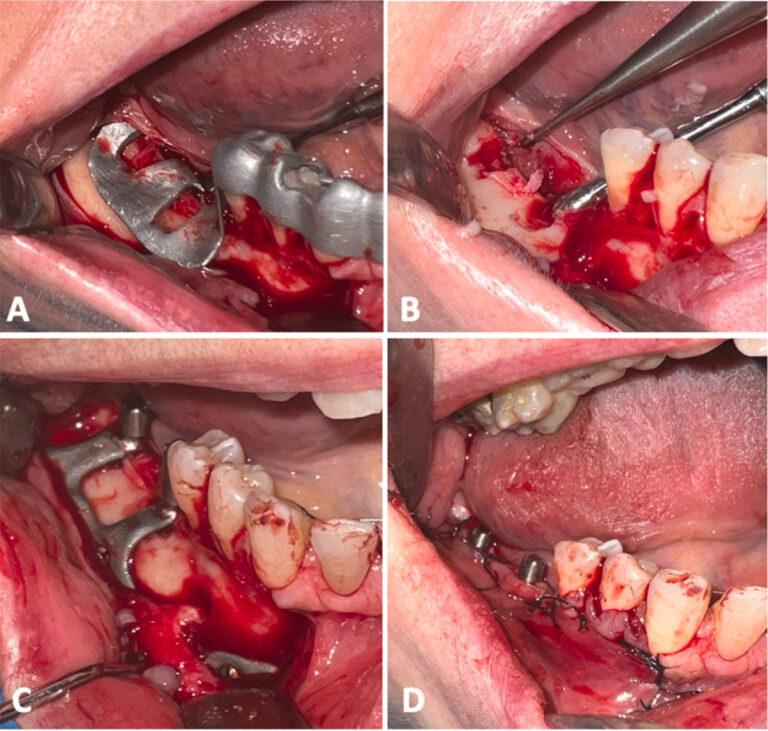
Fig. 2. Case 11 (female, age 54 years): (A) placement of the surgical guide for the preparation of the alveolar ridge; (B) housings for abutments milled into the alveolar bone; (C) subperiosteal implant placed and fixed; (D) surgical access sutured.
Evaluation protocol
All patients underwent clinical and radiological follow-up, and any complications were recorded. The health of the soft tissues around the abutments was evaluated 6 months after the surgery, at delivery of the final prosthesis, and then every 6 months. Peri-implant bleeding on probing (BOP) was measured at six sites per abutment (mesiobuccal, mid-buccal, distobuccal, mesiolingual, mid-lingual, and distolingual) and classified into four grades as described by Mombelli24, from 0 (no bleeding) to 3 (heavy and profuse bleeding upon periodontal probing). The degree of exposure of the implant structure was also assessed, and classified as follows (score range 0–5): no exposure of the structure (score 0), exposure of the vertical arm of the abutment (score 1), exposure of the horizontal arm of the implant (score 2), exposure of the horizontal arm and at least one osteosynthesis screw (score 3), exposure of the implant with mobility (score 4). Each of these scores could be subclassified into two categories based on whether there were associated painful or inflammatory symptoms (subgroup A) or not (subgroup B). Control computed tomography (CT) scans were performed 10 days and 6 months after surgery and then annually. For the purposes of the study, the control CT scans were used to assess the extent of bone resorption beneath the abutments, as described previously for the rehabilitation of the upper jaw16. The degree of resorption for each abutment was assessed by comparing the CT scan at 10 days after surgery (T0) with those obtained at 6 months (T1), 1 year (T2), 2 years (T3), and 3 years (T4) post-surgery.
Fig. 3 Case 9 (female, age 64 years): (A) and (B) show intraoperative views; (C) and (D) show the final prosthesis delivered.
Statistical analysis
The statistical analysis was performed using the open-source software Jamovi, version 2.3.18.0 (accessible online at www.jamovi.org). Data are presented as the absolute number and percentage of the total (categorical variables), or as the mean ± standard deviation (quantitative variables). The differences in the amount of bone resorption beneath the abutments were assessed using the Student t-test for paired samples, with comparison of the values measured at T0 with those measured at T1, T2, T3, and T4. The level of statistical significance was set at P < 0.05 with a 95% confidence interval.
Results
Data from 30 implants performed in 17 patients were included and analysed retrospectively. The sample included 15 female patients and two male patients; their mean age was 60.4 years (range 48–77 years). Supplementary Material Table S1 provides a summary of the patient characteristics. The rehabilitation was unilateral in four cases and bilateral in 13.
The mean duration of surgery was 57.5 minutes (range 31–94 min), with a decreasing trend over time due to the surgeon’s learning curve.
No major complications were encountered during the operation. In three cases, inaccurate fitting of the ridge preparation template was found, due to small bone pre-contacts not detected by the CT scan. After elimination of the pre-contacts with rotary instruments, it was possible to insert the guide and proceed with the preparation of the crest without any further problems. In all but one case, the implant fit was satisfactory.
The main postoperative sequela was oedema, which was reported as moderate by most of the patients and regressed completely within 10 days of surgery. Temporary hypoesthesia of the innervation area of the mental nerve was reported for six implants, all passing below the nerve. This recovered completely in all cases in an average of 3.2 weeks.
No infections or implant loss were detected during follow-up, which ranged from 7 to 53 months (average 22.5 months). In 10 cases, the patient reported chewing discomfort immediately after delivery of the provisional prosthesis. This problem, probably linked to the conditioning of the gingiva or to the transmission of masticatory forces to the periosteum, always regressed within a few weeks.
No particular prosthetic complications were detected either during the provisional phase or final prosthetic phase. The control CBCT scans, performed at 10 days after surgery, at 6 months, and then annually in all cases, did not show displacement of the implants, or loss or loosening of the osteosynthesis screws (Fig. 4).
Analysis of the gap between the implant and the bone beneath the abutments on the CT scans revealed no significant difference between the gap at 10 days postoperative and the gap at 6 months (P = 0.297), 1 year (P = 0.080), 2 years (P = 0.125), or 3 years (P = 0.166) of follow-up (Table 1).
Table 1. Evaluation of bone resorption beneath the abutments (paired samples t-test)
Confronto | n | T0 (mm ± SD) | Tn (mm ± SD) | t-statistic | df | P-value
——————|—-|——————|——————|————-|—–|———
T0 vs T1 (6 mesi) | 71 | 0.379 ± 0.234 | 0.361 ± 0.214 | 1.05 | 70 | 0.297
T0 vs T2 (1 anno) | 52 | 0.392 ± 0.237 | 0.348 ± 0.203 | 1.79 | 51 | 0.080
T0 vs T3 (2 anni) | 20 | 0.410 ± 0.255 | 0.480 ± 0.161 | −1.61 | 19 | 0.125
T0 vs T4 (3 anni) | 15 | 0.413 ± 0.245 | 0.487 ± 0.141 | −1.46 | 14 | 0.166
Legend:
SD: Standard deviation
df: Degrees of freedom
T0: 10 days after surgery
T1: 6 months after surgery
T2: 1 year after surgery
T3: 2 years after surgery
T4: 3 years after surgery
During follow-up, none of the implants presented any type of exposure of the structure (score 0 in 100% of cases). At 6 months, six out of 71 abutments (8.5%) presented BOP (bleeding on probing), scored as 1 (a single isolated spot) in five cases and as 2 (confluent bleeding line) in one case. At 1 year, three out of 52 abutments (5.8%) continued to present BOP, scored as 1 in all cases. BOP was present in 10% of abutments evaluated at 2 years (two abutments out of 20; score 1 in one case and score 2 in the other).
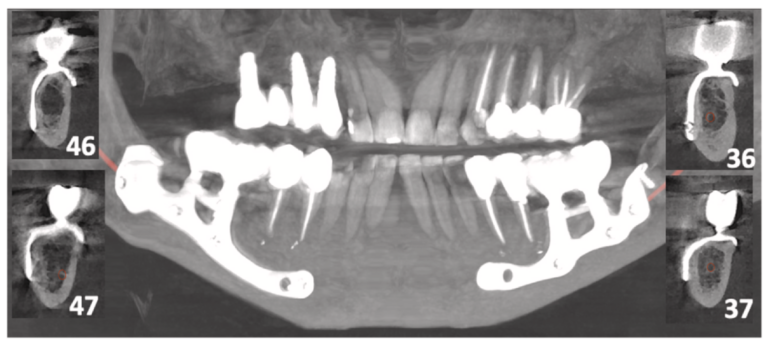
Fig. 4. Case 4 (female, age 59 years): postoperative CBCT scan obtained at 24 months after surgery showing a good fit of the implant and no evidence of bone loss below the abutments.
Discussion
Since 2017, there has been reignited interest in additively manufactured custom-made subperiosteal implants. The evidence on their safety and reliability in the treatment of severe maxillary atrophy has been corroborated by several authors. However, it appears that in recent years, only a few authors have studied the application of this new generation of implants in the mandible, despite the original history of this type of implantology being entirely oriented towards the rehabilitation of the lower jaw.
In an animal study, Bai et al. evaluated 12 custom-made subperiosteal implants placed in the mandible of six beagle dogs, by histology and microcomputed tomography. At 12 weeks after placement, both the implant and the fixation screws showed signs of ossification. The same study also analysed the von Mises stress distribution, highlighting how the implant can disperse the physiological load delivered by chewing on the teeth surfaces without areas of implant structure overload.
Only Mangano et al. have presented results for additively manufactured subperiosteal implants used to rehabilitate atrophic posterior mandibular sectors. The authors reported a 1-year survival rate of 100% in a series of 10 patients. However, the implants used still featured a first-generation design, with the majority of the structure lying just below the surgical wound, rigid fixation placed in the alveolar bone, and no crestal preparation, with the abutments resting on the residual bone profile. Over time, the alveolar bone may undergo further resorption with the loss of screws or decubitus of the implant below the mucosa and subsequent exposure. These were the factors behind the significant rate of loss of first-generation subperiosteal implants over the long term. For this reason, certain factors should be particularly taken into account during implant design planning and surgery.
The buccal portion of the implant should run as far from the surgical wound as possible to prevent bacterial colonization and possible exposure. This is a well-established concept in mandibular fracture fixation, where the complication rate goes from more than 10% for the upper plate to less than 1% for the inferior. Furthermore, in this way it is possible to position the osteosynthesis screws in the areas of greatest resistance of the jaw, following the physiological distribution lines of the masticatory and muscular forces specified by Le Fort and Champy.
For this reason, if the positioning of the abutments allows it, the implant should always run below the emergence of the mental nerve. With this configuration of the implant, complete isolation of the nerve and its adequate protection minimize the risk of neurological deficits, which, if present, recover within a few weeks of the operation. In order to avoid traction damage, tissue retraction should be stopped in the downtime of surgery and the closest screw holes should be placed at least 5–8 mm from the mental foramen.
The literature does not provide data on the minimum number of osteosynthesis screws required. Not less than four screws per implant were used in this series, which is the number that is sufficient for rigid fixation of favourable mandibular fractures. In any case, the osteosynthesis screws only provide primary stability to the implant and allow for immediate loading. The masticatory load is distributed and supported by the bone underlying the plate, as demonstrated by finite element analysis.
The abutments should always be housed in special slots created in the alveolar crest and rest on the basal bone, which is less subject to resorption over time. At present, it is not known what effect the distribution of masticatory loads has on the maintenance of bone trophism in subperiosteal implants in the long term. De Moor et al., in a prospective 1-year follow-up study, detected an average 0.2 mm of crestal bone resorption around subperiosteal implants in the maxilla; this is similar to the resorption reported in the literature for endosseous implants.
Furthermore, removal of the residual alveolar ridge is often necessary to create a regular support base for the abutment, especially in the case of purely horizontal defects. Bone resorption beneath the abutments was evaluated in this study, and no significant variations in the implant/bone gap were observed over time. The gap detected in the postoperative CT scan at 10 days could be due to minor inaccuracies in the CT processing or implant production, the production of the crestal template, or, more likely, a slight over-preparation of the crest during the creation of the slots for the abutments.
This gap was larger in the first implants of the series, where a ball bur was used. In the more recent cases, a larger cylindrical bur was used, which could rest on the edges of the template, allowing more precise preparation. Interestingly, the average gap reduced during the first year after surgery, although not significantly. This could be related to the fact that the implant itself, beneath the abutment, may act as a titanium membrane, preventing the invasion of the gap by connective tissue and allowing the regeneration of the underlying bone.
The absence of significant resorption beneath the implants in this study aligns with findings reported by other authors regarding the upper jaw, where porous titanium implants and screws were used. Similarly, in the cases included in this study, both the implant and the screws were made of grade V porous titanium. This consistency may support the hypothesis that the use of porous titanium devices can mitigate stress shielding effects.
On the lingual side, the connection of the abutments is not possible if the mylohyoid line is very superficial. In fact, its positioning would make it necessary to dissect the muscle from the jaw and, above all, an undercut would be created that could not be crossed by the implant. In the present series, no complications related to the absence of the lingual connection were identified in the short or medium term, but studies on the distribution of loads and finite element analysis will be necessary in the future to evaluate the reliability of this design.
Regarding the possible indications, subperiosteal implants are primarily indicated for graftless rehabilitation of severe atrophy of the posterior mandibular sectors, particularly when the residual bone volumes are insufficient for the use of short implants. Short implants have proven to be a safe and reliable technique, yielding satisfactory long-term results. Compared to subperiosteal implants, they can be placed using guided and less invasive techniques. Like short implants, subperiosteal implants do not provide for a restoration of the bone height, often necessitating compromises in prosthetic aesthetics and requiring the filling of a larger prosthetic space with longer teeth.
However, in many cases, despite a significant vertical bone deficit, the prosthetic space is reduced due to the hyper-eruption of the upper teeth (Fig. 5). In such scenarios, the use of regenerative techniques would result in further reduction of the prosthetic space, making it impossible to accommodate a prosthesis without encroaching on the upper opposing teeth.
The primary limitation of this study is the retrospective design and the relatively small number of patients. In the future, prospective studies and randomized trials will be needed to effectively determine the ideal implant design and the number and location of fixation screws. Although some cases had a follow-up of 4 years, this is still not sufficient to draw definitive conclusions on the long-term results of rehabilitations with subperiosteal implants. It will be essential to monitor the health of the soft tissues over time, as they are crucial for maintaining an adequate seal around the abutments, preventing implant exposure, and avoiding bone resorption.
With the first-generation subperiosteal implants, this process typically occurred at ≥5 years from placement. A benefit of the new generation of subperiosteal implants is the housing of the abutments directly at the level of the basal bone, which tends to resorb less over time. Therefore, the implant should be less affected by possible resorption of the bone surrounding the abutments.
In this study, splitting of the residual keratinized mucosa and careful conditioning of the soft tissues were sufficient to obtain an adequate amount of keratinized gingiva, preventing exposures and inflammation of the gingiva around the abutment. Future studies should evaluate the feasibility of using connective or gingival grafts. Moreover, the sample size was too small, and the results not supported by finite element analysis, to draw definitive clinical conclusions about the shape of the implants and the site of fixation. Large-scale prospective studies will be necessary to obtain meaningful clinical data.
Based on the results of this study, additively manufactured subperiosteal implants could represent a safe and reliable technique for the rehabilitation of severe atrophy of the posterior sectors of the mandible. Pending the results of prospective studies on larger patient series, the choice of implant design should be guided with respect to the principles of osteosynthesis and the biology of the mandibular bone. Studies with longer follow-up will be needed to establish the long-term results of this type of implant-prosthetic rehabilitation.
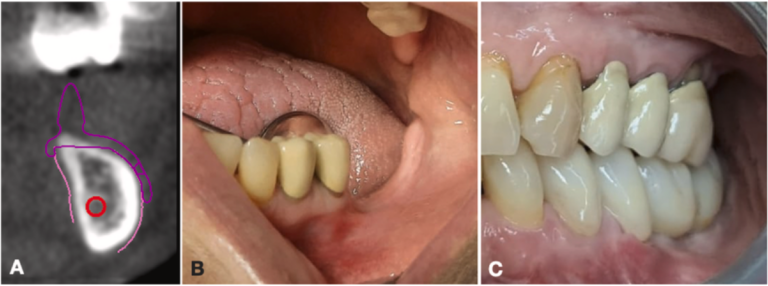
Fig. 5. Case 14 (female, age 49 years): (A) during digital planning, the CT scan showed severe atrophy of the mandible; (B) preoperative view; (C) the definitive prosthesis; note the height of the teeth which, despite the severe vertical atrophy, is not increased, due to the hyper-extrusion of the upper opposing teeth.
References
Raghoebar GM, Meijer HJ, Stellingsma K, Vissking A. Addressing the atrophied mandible: a proposal for a treatment approach involving endosseous implants. Int J Oral Maxillofac Implants 2011;26:607–17.
Baj A, Sollazzo V, Lauritano D, Candotto V, Mancini GE, Gianni AB. Lights and shadows of bone augmentation in severe resorbed mandible in combination with implant dentistry. J Biol Regul Homeost Agents 2016;30:177–82.
Merli M, Moscatelli M, Pagliaro U, Mariotti G, Merli I, Nieri M. Implant prosthetic rehabilitation in partially edentulous patients with bone atrophy. An umbrella review based on systematic reviews of randomized controlled trials. Eur J Oral Implantol 2018;11:261–80.
Dam VV, Trinh HA, Rokaya D, Trinh DH. Bone augmentation for implant placement: recent advances. Int J Dent 2022;2022:8900940.
Elnayef B, Monje A, Gargallo-Albiol J, Galindo-Moreno P, Wang HL, Hernandez-Alfaro F. Vertical ridge augmentation in the atrophic mandible: a systematic review and meta-analysis. Int J Oral Maxillofac Implants 2017;32:291–312.
Sanz-Sachez I, Sanz-Martin I, Ortiz-Vigon A, Molina A, Sanz M. Complications in bone-grafting procedures: classification and management. Periodontol 2000 2022;88:86–102.
Chiapasco M, Cosentini P, Zaniboni M. Bone augmentation procedures in implant dentistry. Int J Oral Maxillofac Implants 2009;24:237–59.
French D, Ofec R, Levin L. Long-term clinical performance of 10,871 dental implants with up to 22 years of follow-up: a cohort study in 4,247 patients. Clin Implant Dent Relat Res 2021;23:289–97.
Terheyden H, Meijer GJ, Raghoebar GM. Vertical bone augmentation and regular implants versus short implants in the vertically deficient posterior mandible: a systematic review and meta-analysis of randomized studies. Int J Oral Maxillofac Surg 2021;50:1249–58.
Xu X, Huang J, Fu X, Kuang Y, Yue H, Song J, Xu L. Short implants versus longer implants in the posterior alveolar region after an observation period of at least five years: a systematic review and meta-analysis. J Dent 2020;100:103386.
Abayev B, Juodzbalys G. Inferior alveolar nerve lateralization and transposition for dental implant placement. Part I: a systematic review of surgical techniques. J Oral Maxillofac Res 2015;6:e2.
Deryabin G, Grybauskas S. Dental implant placement with inferior alveolar nerve repositioning in severely resorbed mandibles: a retrospective multicenter study of implant success and survival rates, and lower lip sensory disturbances. Int J Implant 2021;7:44.
Abayev B, Joudzbalys G. Inferior alveolar nerve lateralization and transposition for dental implant placement. Part II: a systematic review of neurosensory complications. J Oral Maxillofac Res 2015;6:e3.
Vetromilla BM, Moura LB, Sonego CL, Torriani MA, Chagas Jr OL. Complications associated with inferior alveolar nerve repositioning for dental implant placement: a systematic review. Int J Oral Maxillofac Surg 2014;43:1360–6.
Mommaerts MY. Additively manufactured sub-periosteal jaw implants. Int J Oral Maxillofac Surg 2017;46:938–40.
Van den Borre C, Rinaldi M, De Neef B, et al. Radiographic evaluation of bone remodeling after additively manufactured subperiosteal jaw implantation (AMSJI) in the maxilla: a one-year follow-up study. J Clin Med 2021;10:3542.
Van den Borre C, Rinaldi M, De Neef B, et al. Patient and clinician-reported outcomes for the additively manufactured sub-periosteal jaw implant (AMSJI) in the maxilla: a prospective multicentre one-year follow-up study. Int J Oral Maxillofac Surg 2022;51:243–50.
De Moor E, Huys SEF, van Lenthe GH, Mommaerts MY, Vander Sloten J. Mechanical evaluation of a patient-specific additively manufactured subperiosteal jaw implant (AMSJI) using finite-element analysis. Int J Oral Maxillofac Surg 2022;51:405–11.
Mommaerts MY. Evolutionary steps in the design and biofunctionalization of the additively manufactured sub-periosteal jaw implant ‘AMSJI’ for the maxilla. Int J Oral Maxillofac Surg 2019;48:108–14.
Mounir M, Atef M, Abou-Elfetouh A, Hakam MM. Titanium and polyether ether ketone (PEEK) patient-specific sub-periosteal implants: two novel approaches for rehabilitation of the severely atrophic anterior maxillary ridge. Int J Oral Maxillofac Surg 2018;47:658–64.
Dimitroulis G, Gupta B, Wilson I, Hart C. The atrophic edentulous alveolus. A preliminary study on a new generation of subperiosteal implants. Oral Maxillofac Surg 2023;27:69–78.
Nemtoi A, Covrig V, Nemtoi A, et al. Custom-made direct metal laser sintering titanium subperiosteal implants in oral and maxillofacial surgery for severe bone-deficient patients—a pilot study. Diagnostics 2022;12:2531.
von Elm E, Altman DG, Egger M, et al. STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8.
Mombelli A. Clinical parameters: biological validity and clinical utility. Periodontol 2000 2005;39:30–9.
Linkow LI. Evolutionary design trends in the mandibular subperiosteal implant. J Oral Implantol 1984;11:402–38.
Linkow LI, Wagner JR, Chanavaz M. Tripodal mandibular subperiosteal implant: basic sciences, operational procedures, and clinical data. J Oral Implantol 1998;24:16–36.
Bai L, Zheng L, Ji P, et al. Additively manufactured lattice-like subperiosteal implants for rehabilitation of the severely atrophic ridge. ACS Biomater Sci Eng 2022;8:912–20.
Mangano C, Bianchi A, Mangano FG, et al. Custom-made 3D printed subperiosteal titanium implants for the prosthetic restoration of the atrophic posterior mandible of elderly patients: a case series. 3D Print Med 2020;6(1).
Falci SGM, de Souza GM, Fernandes IA, et al. Complications after different methods for fixation of mandibular angle fractures: network meta-analysis of randomized controlled trials. Int J Oral Maxillofac Surg 2021;50:1450–63.
Shah A, Patel A, Steinbacher D. Soft tissue coverage for mandibular fractures using two miniplates. Craniomaxillofac Trauma Reconstr 2012;5:253–4.
Champy M, Loddé JP, Schmitt R, Jaeger JH, Muster D. Mandibular osteosynthesis by miniature screwed plates via a buccal approach. J Maxillofac Surg 1978;6:14–21.
Sáenz-Ravello G, Ossandón-Zúñiga B, Muñoz-Meza V, et al. Short implants compared to regular dental implants after bone augmentation in the atrophic posterior mandible: umbrella review and meta-analysis of success outcomes. Int J Implant Dent 2023;9:18.
Post Recenti
Implant-prosthetic rehabilitation of the atrophic posterior mandible with additively manufactured custom-made subperiosteal implants: a cohort …
Custom Fabricated Subperiosteal Implants for Sectional Rehabilitation of Severely Atrophic Maxillae: A Technical Note Luigi …
Bone Remodeling Around Implants with Different Macro-Design Placed in Post-Extraction Sockets: A Cone-Beam Computed Tomography …
Full-arch rehabilitation of severely atrophic maxilla with additively manufactured custom-made subperiosteal implants: A multicenter retrospective …